The driving forces of cell division
 At the onset of division the cell forms a spindle, a micro-machine made of microtubules, which divide the chromosomes by pulling on kinetochores, protein complexes on the chromosome. The central question in the field is how accurate chromosome segregation results from the interactions between kinetochores, microtubules and the associated proteins.
At the onset of division the cell forms a spindle, a micro-machine made of microtubules, which divide the chromosomes by pulling on kinetochores, protein complexes on the chromosome. The central question in the field is how accurate chromosome segregation results from the interactions between kinetochores, microtubules and the associated proteins.
According to the current paradigm, the forces on kinetochores are produced by k-fibers, bundles of microtubules extending between the spindle pole and the kinetochore. The proposed project is built upon a hypothesis that a new class of microtubules, which we term bridging microtubules, bridge sister kinetochores.
We are taking an interdisciplinary approach by combining laser microsurgery with genetic perturbations, quantitative measurements of the responses and comparison with theoretical models. Understanding the role of bridging microtubules in force generation and chromosome movements will not only shed light on the mechanism of chromosome segregation, but may also increase the potential of mitotic anticancer strategies, as the spindle is a major target for chemotherapy.

Research area: Cellular and Developmental Biology
Researcher: Prof Iva Tolić
Host Institution: Ruđer Bošković Institute, Zagreb, Croatia
Project: A new class of microtubules in the spindle exerting forces on kinetochores (NewSpindleForce)
ERC call: Consolidator Grant 2014
ERC funding: € 2.15 million for five years
Featured research papers
The mitotic spindle is chiral due to torques within microtubule bundles.
Maja Novak, Bruno Polak, Juraj Simunić, Zvonimir Boban, Barbara Kuzmić, Andreas Thomae, Iva M. Tolić, Nenad Pavin. Nat Commun, 9(1): 3571 (2018). PDF | web
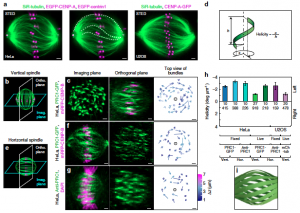 Mitosis relies on forces generated in the spindle, a micro-machine composed of microtubules and associated proteins. Forces are required for the congression of chromosomes to the metaphase plate and their separation in anaphase. However, besides forces, torques may exist in the spindle, yet they have not been investigated. Here we show that the spindle is chiral. Chirality is evident from the finding that microtubule bundles in human spindles follow a left-handed helical path, which cannot be explained by forces but rather by torques. Kinesin-5 (Kif11/Eg5) inactivation abolishes spindle chirality. Our theoretical model predicts that bending and twisting moments may generate curved shapes of bundles. We found that bundles turn by about −2 deg µm−1 around the spindle axis, which we explain by a twisting moment of roughly −10 pNµm. We conclude that torques, in addition to forces, exist in the spindle and determine its chiral architecture.
Mitosis relies on forces generated in the spindle, a micro-machine composed of microtubules and associated proteins. Forces are required for the congression of chromosomes to the metaphase plate and their separation in anaphase. However, besides forces, torques may exist in the spindle, yet they have not been investigated. Here we show that the spindle is chiral. Chirality is evident from the finding that microtubule bundles in human spindles follow a left-handed helical path, which cannot be explained by forces but rather by torques. Kinesin-5 (Kif11/Eg5) inactivation abolishes spindle chirality. Our theoretical model predicts that bending and twisting moments may generate curved shapes of bundles. We found that bundles turn by about −2 deg µm−1 around the spindle axis, which we explain by a twisting moment of roughly −10 pNµm. We conclude that torques, in addition to forces, exist in the spindle and determine its chiral architecture.
Microtubule Sliding within the Bridging Fiber Pushes Kinetochore Fibers Apart to Segregate Chromosomes.
Kruno Vukušić, Renata Buđa, Agneza Bosilj, Ana Milas, Nenad Pavin, Iva M. Tolić. Dev Cell, 43(1): 11-23 (2017). PDF | web
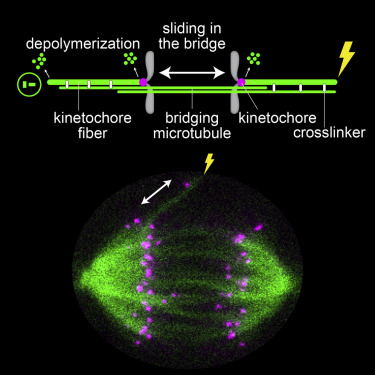 During cell division, mitotic spindle microtubules segregate chromosomes by exerting forces on kinetochores. What forces drive chromosome segregation in anaphase remains a central question. The current model for anaphase in human cells includes shortening of kinetochore fibers and separation of spindle poles. Both processes require kinetochores to be linked with the poles. Here we show, by combining laser ablation, photoactivation, and theoretical modeling, that kinetochores can separate without any attachment to one spindle pole. This separation requires the bridging fiber, a microtubule bundle that connects sister kinetochore fibers. Bridging fiber microtubules in intact spindles slide apart with kinetochore fibers, indicating strong crosslinks between them. We conclude that sliding of microtubules within the bridging fibers drives pole separation and pushes kinetochore fibers poleward by the friction of passive crosslinks between these fibers. Thus, sliding within the bridging fiber works together with the shortening of kinetochore fibers to segregate chromosomes.
During cell division, mitotic spindle microtubules segregate chromosomes by exerting forces on kinetochores. What forces drive chromosome segregation in anaphase remains a central question. The current model for anaphase in human cells includes shortening of kinetochore fibers and separation of spindle poles. Both processes require kinetochores to be linked with the poles. Here we show, by combining laser ablation, photoactivation, and theoretical modeling, that kinetochores can separate without any attachment to one spindle pole. This separation requires the bridging fiber, a microtubule bundle that connects sister kinetochore fibers. Bridging fiber microtubules in intact spindles slide apart with kinetochore fibers, indicating strong crosslinks between them. We conclude that sliding of microtubules within the bridging fibers drives pole separation and pushes kinetochore fibers poleward by the friction of passive crosslinks between these fibers. Thus, sliding within the bridging fiber works together with the shortening of kinetochore fibers to segregate chromosomes.
PRC1-labeled microtubule bundles and kinetochore pairs show one-to-one association in metaphase.
Bruno Polak, Patrik Risteski, Sonja Lesjak, Iva M. Tolić. EMBO Rep, 18(2): 217–230 (2017). PDF | web
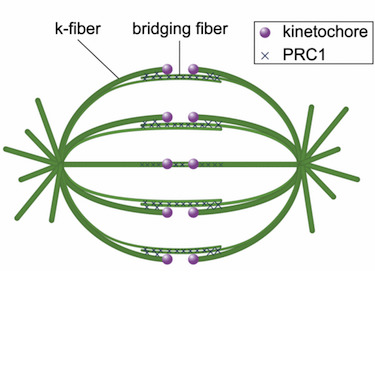 In the mitotic spindle, kinetochore microtubules form k‐fibers, whereas overlap or interpolar microtubules form antiparallel arrays containing the cross‐linker protein regulator of cytokinesis 1 (PRC1). We have recently shown that an overlap bundle, termed bridging fiber, links outermost sister k‐fibers. However, the relationship between overlap bundles and k‐fibers throughout the spindle remained unknown. Here, we show that in a metaphase spindle more than 90% of overlap bundles act as a bridge between sister k‐fibers. We found that the number of PRC1‐GFP‐labeled bundles per spindle is nearly the same as the number of kinetochore pairs. Live‐cell imaging revealed that kinetochore movement in the equatorial plane of the spindle is highly correlated with the movement of the coupled PRC1‐GFP‐labeled fiber, whereas the correlation with other fibers decreases with increasing distance. Analysis of endogenous PRC1 localization confirmed the results obtained with PRC1‐GFP. PRC1 knockdown reduced the bridging fiber thickness and interkinetochore distance throughout the spindle, suggesting a function of PRC1 in bridging microtubule organization and force balance in the metaphase spindle.
In the mitotic spindle, kinetochore microtubules form k‐fibers, whereas overlap or interpolar microtubules form antiparallel arrays containing the cross‐linker protein regulator of cytokinesis 1 (PRC1). We have recently shown that an overlap bundle, termed bridging fiber, links outermost sister k‐fibers. However, the relationship between overlap bundles and k‐fibers throughout the spindle remained unknown. Here, we show that in a metaphase spindle more than 90% of overlap bundles act as a bridge between sister k‐fibers. We found that the number of PRC1‐GFP‐labeled bundles per spindle is nearly the same as the number of kinetochore pairs. Live‐cell imaging revealed that kinetochore movement in the equatorial plane of the spindle is highly correlated with the movement of the coupled PRC1‐GFP‐labeled fiber, whereas the correlation with other fibers decreases with increasing distance. Analysis of endogenous PRC1 localization confirmed the results obtained with PRC1‐GFP. PRC1 knockdown reduced the bridging fiber thickness and interkinetochore distance throughout the spindle, suggesting a function of PRC1 in bridging microtubule organization and force balance in the metaphase spindle.
Overlap microtubules link sister k-fibres and balance the forces on bi-oriented kinetochores.
Janko Kajtez, Anastasia Solomatina, Maja Novak, Bruno Polak, Kruno Vukušić, Jonas Rüdiger, Gheorghe Cojoc, Ana Milas, Ivana Šumanovac Šestak, Patrik Risteski, Federica Tavano, Anna H. Klemm, Emanuele Roscioli, Julie Welburn, Daniela Cimini, Matko Glunčić, Nenad Pavin, Iva M. Tolić. Nat Commun, 7: 10298 (2016). PDF | web
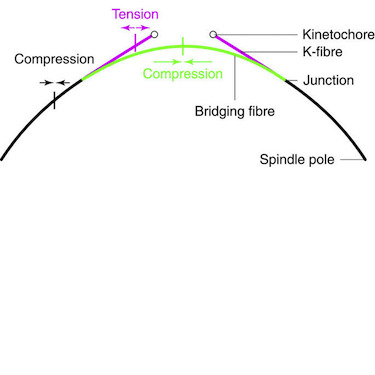 During metaphase, forces on kinetochores are exerted by k-fibres, bundles of microtubules that end at the kinetochore. Interestingly, non-kinetochore microtubules have been observed between sister kinetochores, but their function is unknown. Here we show by laser-cutting of a k-fibre in HeLa and PtK1 cells that a bundle of non-kinetochore microtubules, which we term ‘bridging fibre’, bridges sister k-fibres and balances the interkinetochore tension. We found PRC1 and EB3 in the bridging fibre, suggesting that it consists of antiparallel dynamic microtubules. By using a theoretical model that includes a bridging fibre, we show that the forces at the pole and at the kinetochore depend on the bridging fibre thickness. Moreover, our theory and experiments show larger relaxation of the interkinetochore distance for cuts closer to kinetochores. We conclude that the bridging fibre, by linking sister k-fibres, withstands the tension between sister kinetochores and enables the spindle to obtain a curved shape.
During metaphase, forces on kinetochores are exerted by k-fibres, bundles of microtubules that end at the kinetochore. Interestingly, non-kinetochore microtubules have been observed between sister kinetochores, but their function is unknown. Here we show by laser-cutting of a k-fibre in HeLa and PtK1 cells that a bundle of non-kinetochore microtubules, which we term ‘bridging fibre’, bridges sister k-fibres and balances the interkinetochore tension. We found PRC1 and EB3 in the bridging fibre, suggesting that it consists of antiparallel dynamic microtubules. By using a theoretical model that includes a bridging fibre, we show that the forces at the pole and at the kinetochore depend on the bridging fibre thickness. Moreover, our theory and experiments show larger relaxation of the interkinetochore distance for cuts closer to kinetochores. We conclude that the bridging fibre, by linking sister k-fibres, withstands the tension between sister kinetochores and enables the spindle to obtain a curved shape.
Featured reviews
Kruno Vukušić, Renata Buđa, Iva M. Tolić.
Force-generating mechanisms of anaphase in human cells.
J Cell Sci, 132: jcs231985 (2019). PDF | web
What forces drive chromosome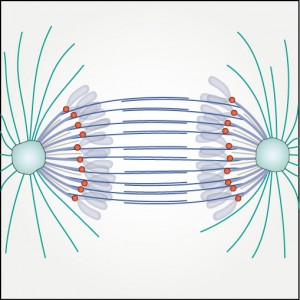 segregation remains one of the most challenging questions in cell division. Even though the duration of anaphase is short, it is of utmost importance for genome fidelity that no mistakes are made. Seminal studies in model organisms have revealed different mechanisms operating during chromosome segregation in anaphase, but the translation of these mechanisms to human cells is not straightforward. Recent work has shown that kinetochore fiber depolymerization during anaphase A is largely motor independent, whereas spindle elongation during anaphase B is coupled to sliding of interpolar microtubules in human cells. In this Review, we discuss the current knowledge on the mechanisms of force generation by kinetochore, interpolar and astral microtubules. By combining results from numerous studies, we propose a comprehensive picture of the role of individual force-producing and -regulating proteins. Finally, by linking key concepts of anaphase to most recent data, we summarize the contribution of all proposed mechanisms to chromosome segregation and argue that sliding of interpolar microtubules and depolymerization at the kinetochore are the main drivers of chromosome segregation during early anaphase in human cells.
segregation remains one of the most challenging questions in cell division. Even though the duration of anaphase is short, it is of utmost importance for genome fidelity that no mistakes are made. Seminal studies in model organisms have revealed different mechanisms operating during chromosome segregation in anaphase, but the translation of these mechanisms to human cells is not straightforward. Recent work has shown that kinetochore fiber depolymerization during anaphase A is largely motor independent, whereas spindle elongation during anaphase B is coupled to sliding of interpolar microtubules in human cells. In this Review, we discuss the current knowledge on the mechanisms of force generation by kinetochore, interpolar and astral microtubules. By combining results from numerous studies, we propose a comprehensive picture of the role of individual force-producing and -regulating proteins. Finally, by linking key concepts of anaphase to most recent data, we summarize the contribution of all proposed mechanisms to chromosome segregation and argue that sliding of interpolar microtubules and depolymerization at the kinetochore are the main drivers of chromosome segregation during early anaphase in human cells.
Helical twist and rotational forces in the mitotic spindle.
Iva M.Tolić, Maja Novak, Nenad Pavin. Biomolecules, 9(4): 132 (2019). PDF | web
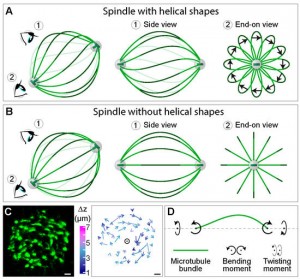 The mitotic spindle segregates chromosomes into two daughter cells during cell division. This process relies on the precise regulation of forces acting on chromosomes as the cell progresses through mitosis. The forces in the spindle are difficult to directly measure using the available experimental techniques. Here, we review the ideas and recent advances of how forces can be determined from the spindle shape. By using these approaches, it has been shown that tension and compression coexist along a single kinetochore fiber, which are balanced by a bridging fiber between sister kinetochore fibers. An extension of this approach to three dimensions revealed that microtubule bundles have rich shapes, and extend not simply like meridians on the Earth’s surface but, rather, twisted in a helical manner. Such complex shapes are due to rotational forces, which, in addition to linear forces, act in the spindle and may be generated by motor proteins such as kinesin-5. These findings open new questions for future studies, to understand the mechanisms of rotational forces and reveal their biological roles in cells.
The mitotic spindle segregates chromosomes into two daughter cells during cell division. This process relies on the precise regulation of forces acting on chromosomes as the cell progresses through mitosis. The forces in the spindle are difficult to directly measure using the available experimental techniques. Here, we review the ideas and recent advances of how forces can be determined from the spindle shape. By using these approaches, it has been shown that tension and compression coexist along a single kinetochore fiber, which are balanced by a bridging fiber between sister kinetochore fibers. An extension of this approach to three dimensions revealed that microtubule bundles have rich shapes, and extend not simply like meridians on the Earth’s surface but, rather, twisted in a helical manner. Such complex shapes are due to rotational forces, which, in addition to linear forces, act in the spindle and may be generated by motor proteins such as kinesin-5. These findings open new questions for future studies, to understand the mechanisms of rotational forces and reveal their biological roles in cells.
Mitotic spindle: kinetochore fibers hold on tight to interpolar bundles.
Iva M. Tolić. Eur Biophys J, 47: 191-203 (2018). PDF | web
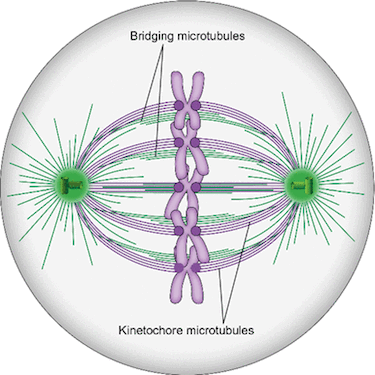 Spindle microtubules can be divided into three major classes: kinetochore microtubules, which form k-fibers ending at the kinetochore; interpolar microtubules, which extend from the opposite sides of the spindle and interact in the middle; and astral microtubules, which extend towards the cell cortex. Recent work in human cells has shown a close relationship between interpolar and kinetochore microtubules, where interpolar bundles are attached laterally to kinetochore fibers almost all along their length, acting as a bridge between sister k-fibers. Most of the interpolar bundles are attached to a pair of sister kinetochore fibers and vice versa. Thus, the spindle is made of modules consisting of a pair of sister kinetochore fibers and a bundle of interpolar microtubules that connects them. These interpolar bundles, termed bridging fibers, balance the forces acting at kinetochores and support the rounded shape of the spindle during metaphase. This review discusses the structure, function, and formation of kinetochore fibers and interpolar bundles, with an emphasis on how they interact. Their connections have an impact on the force balance in the spindle and on chromosome movement during mitosis because the forces in interpolar bundles are transmitted to kinetochore fibers and hence to kinetochores through these connections.
Spindle microtubules can be divided into three major classes: kinetochore microtubules, which form k-fibers ending at the kinetochore; interpolar microtubules, which extend from the opposite sides of the spindle and interact in the middle; and astral microtubules, which extend towards the cell cortex. Recent work in human cells has shown a close relationship between interpolar and kinetochore microtubules, where interpolar bundles are attached laterally to kinetochore fibers almost all along their length, acting as a bridge between sister k-fibers. Most of the interpolar bundles are attached to a pair of sister kinetochore fibers and vice versa. Thus, the spindle is made of modules consisting of a pair of sister kinetochore fibers and a bundle of interpolar microtubules that connects them. These interpolar bundles, termed bridging fibers, balance the forces acting at kinetochores and support the rounded shape of the spindle during metaphase. This review discusses the structure, function, and formation of kinetochore fibers and interpolar bundles, with an emphasis on how they interact. Their connections have an impact on the force balance in the spindle and on chromosome movement during mitosis because the forces in interpolar bundles are transmitted to kinetochore fibers and hence to kinetochores through these connections.
Mitotic Spindle Assembly: Building the Bridge between Sister K-Fibers.
Juraj Simunić, Iva M. Tolić. Trends Biochem Sci, 41(10): 824–833 (2016). PDF | web
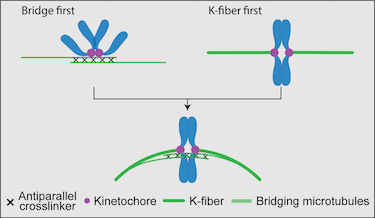
The mitotic spindle performs the task of physically dividing the genetic material between the newly formed daughter cells. To achieve this, bundles of microtubules and associated proteins orchestrate forces that spatially organize and then separate the chromosomes. In the classic view of the spindle, the kinetochore microtubules (k-fibers) are tensed and, thus, straight, whereas interpolar bundles are curved and do not interact with k-fibers close to the spindle equator. The updated view of the spindle depicts k-fibers as curved and interacting with newly identified interpolar bundles, called bridging fibers, along their length. In this Opinion, we propose and discuss scenarios for the origin of this structure in the context of known spindle assembly mechanisms.
Self-Organization and Forces in the Mitotic Spindle.
Nenad Pavin, Iva M. Tolić. Annu Rev Biophys, 45: 279–298 (2016). PDF | web
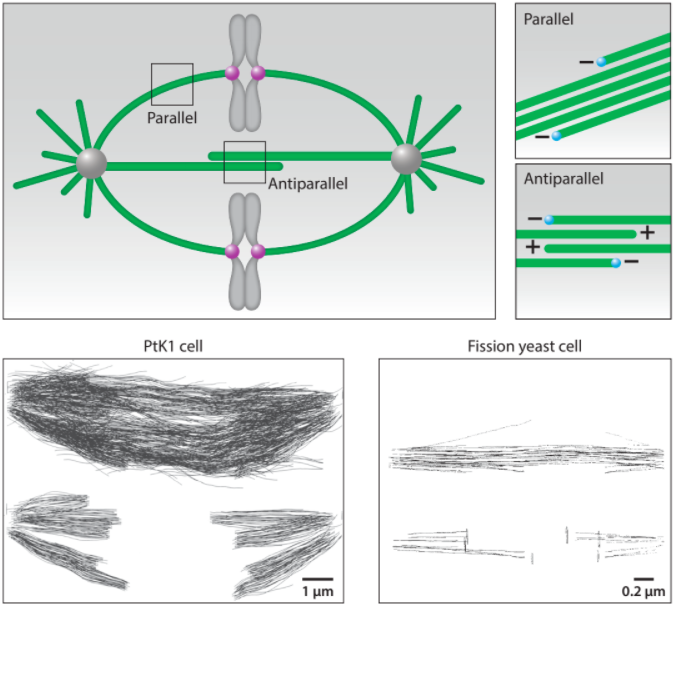
At the onset of division, the cell forms a spindle, a precise self-constructed micromachine composed of microtubules and the associated proteins, which divides the chromosomes between the two nascent daughter cells. The spindle arises from self-organization of microtubules and chromosomes, whose different types of motion help them explore the space and eventually approach and interact with each other. Once the interactions between the chromosomes and the microtubules have been established, the chromosomes are moved to the equatorial plane of the spindle and ultimately toward the opposite spindle poles. These transport processes rely on directed forces that are precisely regulated in space and time. In this review, we discuss how microtubule dynamics and their rotational movement drive spindle self-organization, as well as how the forces acting in the spindle are generated, balanced, and regulated.
Other publications
Optogenetic control of PRC1 reveals its role in chromosome alignment on the spindle by overlap length-dependent forces.
Jagrić, Mihaela; Risteski, Patrik; Martinčić, Jelena; Milas, Ana; Tolić, Iva M.
eLife, 10: e61170 (2021). PDF | web
Mechanobiology of the mitotic spindle.
Pavin, Nenad; Tolić, Iva M.
Dev Cell, 56(2):192-201 (2021). PDF | web
Mitotic spindle: Lessons from theoretical modeling.
Tolić, Iva M.; Pavin, Nenad.
Mol Biol Cell, 32(3): 218–222 (2021). PDF | web
Expansion microscopy of the mitotic spindle.
Ponjavić, Ivana; Vukušić, Kruno; Tolić, Iva M.
Methods Cell Biol, 161: 247-274 (2021). PDF | web
Force-generating mechanisms of anaphase in human cells.
Vukušić, Kruno; Buđa, Renata; Tolić, Iva M.
J Cell Sci, 132: jcs231985 (2019). PDF | web
Pivot-and-bond model explains microtubule bundle formation.
Prelogović, Marcel; Winters, Lora; Milas, Ana; Tolić, Iva M.; Pavin, Nenad.
Phys Rev E, 100: 012403 (2019). PDF | web
Meiotic spindle has a soft spot.
Simunić, Juraj; Tolić, Iva M.
Dev Cell, 49(2): 159-160 (2019). PDF | web
Pivoting of microtubules driven by minus-end-directed motors leads to spindle assembly.
Winters, Lora; Ban, Ivana; Prelogović, Marcel; Kalinina, Iana M.; Pavin, Nenad; Tolić, Iva M.
BMC Biology, 17: 42 (2019). PDF |web
Optogenetic reversible knocksideways, laser ablation and photoactivation on the mitotic spindle in human cells.
Ana Milas, Mihaela Jagrić, Jelena Martinčić, Iva M. Tolić.
Methods Cell Biol, 145: 191-215 (2018). PDF | web
Dissection and characterization of microtubule bundles in the mitotic spindle using femtosecond laser ablation.
Renata Buđa, Kruno Vukušić, Iva M. Tolić.
Methods Cell Biol, 139: 81–101 (2017). PDF | web
Bridging the gap between sister kinetochores.
Iva M. Tolić, Nenad Pavin.
Cell Cycle, 15(9): 1169-1170 (2016). PDF | web
TrendsTalk. Iva Tolić: Movements inside Cells and across Countries.
Iva M. Tolić.
Trends Biochem Sci, 41(10): 815–816 (2016). PDF | web
Relaxation of interkinetochore tension after severing of a k-fiber depends on the length of the k-fiber stub.
Ana Milas, Iva M. Tolić.
Matters Select, March 22 (2016). PDF | web

