Oscillatory dynamics of the cytoskeleton
Oscillations in the cytoskeleton are of general importance in cell biology as a mean of spatial and temporal regulation of the cellular organization, cell division and motility. The project OSCITON focuses on two oscillatory processes. First, we will investigate the mechanism of microtubule-driven kinetochore oscillations during mitosis, and the role of kinesin-8 motors in this process. Second, we will study the oscillatory repolarization of Dictyostelium cells during random migration. This interdisciplinary project is designed to optimize the use of resources and bring together researchers with the expertise in a range of disciplines, including molecular and cellular biology, biochemistry, microscopy, and theoretical biophysics.
2015-2019 OSCITON
Research area: Cell biology
Researcher: Prof Iva Tolić
Host Institution: Ruđer Bošković Institute, Zagreb, Croatia
Project: Oscilatory dynamics of the cytoskeleton (OSCITON)
HRZZ funding: HRK 1.000.000,00
Featured publication
Optogenetic reversible knocksideways, laser ablation and photoactivation on the mitotic spindle in human cells.
Ana Milas, Mihaela Jagrić, Jelena Martinčić, Iva M. Tolić. Methods Cell Biol, 145: 191-215 (2018). PDF | web
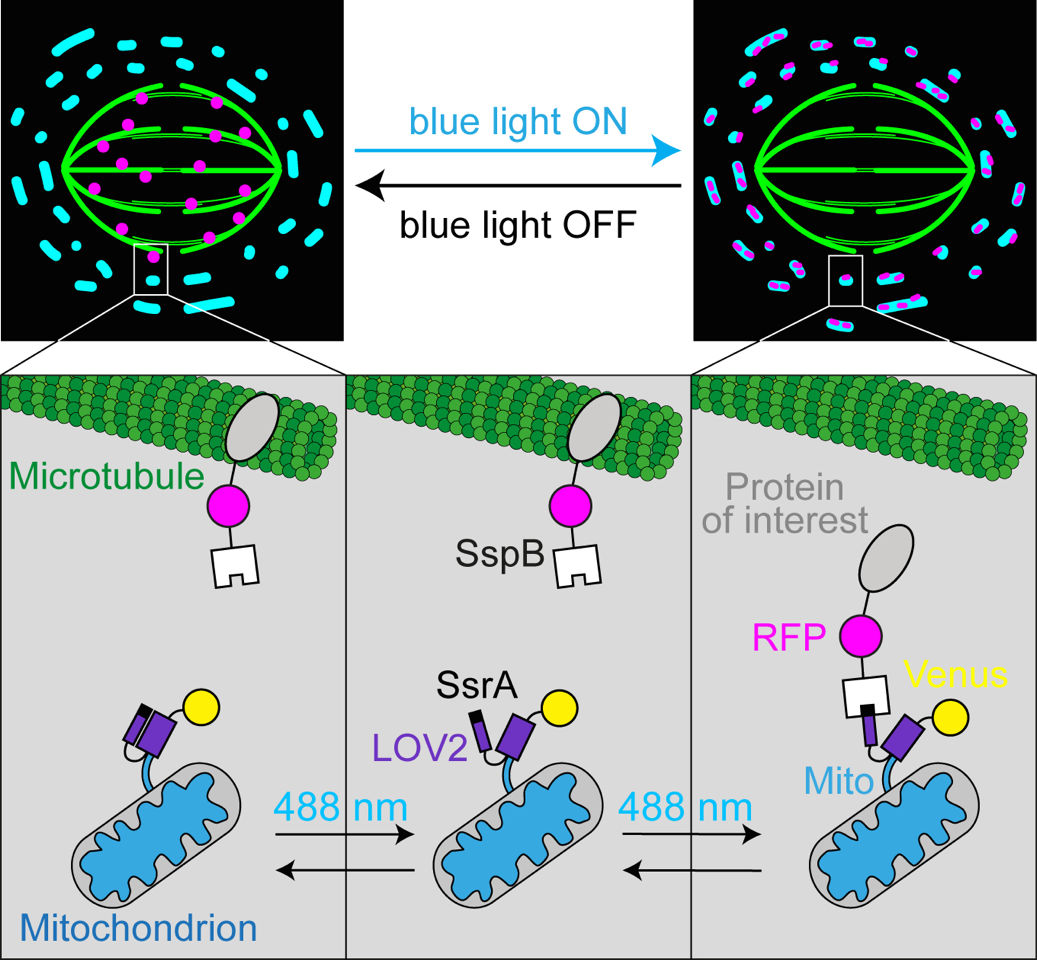 At the onset of mitosis, cells assemble the mitotic spindle, a dynamic micromachine made of microtubules and associated proteins. Although most of these proteins have been identified, it is still unknown how their collective behavior drives spindle formation and function. Over the last decade, RNA interference has been the main tool for revealing the role of spindle proteins. However, the effects of this method are evident only after a longer time period, leading to difficulties in the interpretation of phenotypes. Optogenetics is a novel technology that enables fast, reversible, and precise control of protein activity by utilization of light. In this chapter, we present an optogenetic knocksideways method for rapid and reversible translocation of proteins from the mitotic spindle to mitochondria using blue light. Furthermore, we discuss other optical approaches, such as laser ablation of microtubule bundles in the spindle and creation of reference marks on the bundles by photoactivation of photoactivatable GFP. Finally, we show how different optical perturbations can be combined in order to acquire deeper understanding of the mechanics of mitosis.
At the onset of mitosis, cells assemble the mitotic spindle, a dynamic micromachine made of microtubules and associated proteins. Although most of these proteins have been identified, it is still unknown how their collective behavior drives spindle formation and function. Over the last decade, RNA interference has been the main tool for revealing the role of spindle proteins. However, the effects of this method are evident only after a longer time period, leading to difficulties in the interpretation of phenotypes. Optogenetics is a novel technology that enables fast, reversible, and precise control of protein activity by utilization of light. In this chapter, we present an optogenetic knocksideways method for rapid and reversible translocation of proteins from the mitotic spindle to mitochondria using blue light. Furthermore, we discuss other optical approaches, such as laser ablation of microtubule bundles in the spindle and creation of reference marks on the bundles by photoactivation of photoactivatable GFP. Finally, we show how different optical perturbations can be combined in order to acquire deeper understanding of the mechanics of mitosis.
Other publications
Optogenetic control of PRC1 reveals its role in chromosome alignment on the spindle by overlap length-dependent forces.
Jagrić, Mihaela; Risteski, Patrik; Martinčić, Jelena; Milas, Ana; Tolić, Iva M.
eLife, 10: e61170 (2021). PDF | web
Expansion microscopy of the mitotic spindle.
Ponjavić, Ivana; Vukušić, Kruno; Tolić, Iva M.
Methods Cell Biol, 161: 247-274 (2021). PDF | web
Force-generating mechanisms of anaphase in human cells.
Kruno Vukušić, Renata Buđa, Iva M. Tolić.
J Cell Sci, 132: jcs231985 (2019). PDF | web
Helical twist and rotational forces in the mitotic spindle.
Iva M.Tolić, Maja Novak, Nenad Pavin.
Biomolecules, 9(4): 132 (2019). PDF | web
The mitotic spindle is chiral due to torques within microtubule bundles.
Maja Novak, Bruno Polak, Juraj Simunić, Zvonimir Boban, Barbara Kuzmić, Andreas Thomae, Iva M. Tolić, Nenad Pavin.
Nat Commun, 9(1): 3571 (2018). PDF | web
Metaphase kinetochore movements are regulated by kinesin-8 motors and microtubule dynamic instability.
Anna H. Klemm, Agneza Bosilj, Matko Glunčić, Nenad Pavin, Iva M. Tolić.
Mol Biol Cell, 29(11): 1332-1345 (2018). PDF | web
